Bowen s Reaction Series Continuous Vs Discontinuous
Bowen's reaction series is based on observations and experiments of natural rocks, the crystallization sequence of typical basaltic magma change as they cool. It is a sorting tool according to the temperature at which they crystallize common magmatic silicate minerals. Bowen's Reaction Series describes temperatures at which different common silicate minerals change from liquid to solid phase (or solid to liquid). Petrologist Norman Bowen (1887-1956) conducted decades of melting experiments to support granite theory in the early 1900s. He found that a basaltic meltdown slowly cooled, and minerals formed crystals in a certain order.
This reaction series implies that from a single "parental magma" all the various kinds of igneous rocks can be derived by Magmatic Differentiation (see below)
Bowen's principles
- As a melt cools, the minerals in the thermodynamic equilibrium with the melt crystallize (the dissolution equals crystallization; if there is no equilibrium either crystallization will dominate [supersaturation], or dissolution [under saturated]).
- As the melt continues to cool and the minerals crystallize, it will change the melt composition.
- Previously formed crystals will not be in equilibrium with this solution and will be dissolved again to form new minerals. In other words: these crystals react with the melt to form new crystals, hence the name reaction series.
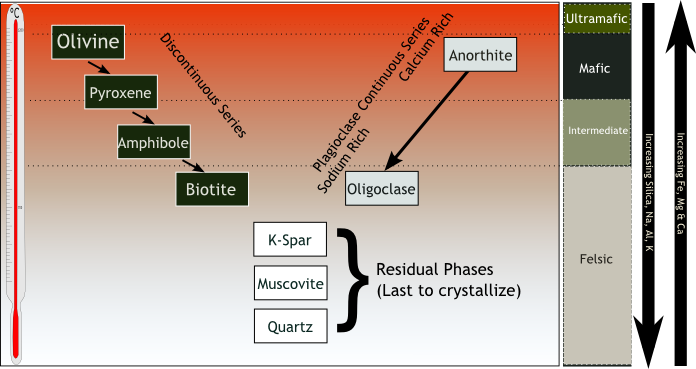
- Common minerals of igneous rocks can be arranged in two series as a continuous reaction sequence of feldspar and as a discontinuous reaction sequence of ferromagnesian minerals (olivine, pyroxene, hornblende, and biotite).
- This sequence of reactions implies that all various magmatic rocks can be obtained by Magmatic Differentiation from a single "parent magma".
Generally speaking, higher temperature minerals have a higher proportion of iron and magnesium and are therefore considered mafic. Low temperature minerals are associated with the opposite end of the composite spectrum (low in iron and magnesium, higher in silicon and oxygen) and considered to be felsic. Some minerals are clearly mafic, some are clearly felsic, and some fall between these two extremes.
Common mafic minerals include olivine, pyroxene , amphibole, and biotite mica and plagioclase feldspar. Common felsic minerals include quartz, muscovite mica and orthoclase feldspar. Different magma compositions clearly result in different magmatic rocks.
Another factor contributing to the differences in igneous rocks is related to the time taken for the crystallization of magma. In general, the faster the cooling rate (if extrusion is common for volcanic rocks), the smaller the mineral grains obtained. A slower cooling history (often intrusive) results in a coarser-grained rock.
Magmas of intermediate compounds cause crystallization of intermediate minerals (in fact a mixture of medium range minerals: amphibole and both feldspar species), common magmatic rocks andesite (extrusion) and diorite (intrusive).
Simply put, the minerals found at the first temperature that crystallize in a magma mass are the most unstable on the Earth's surface and go to air as quickly as the surface is the most different from the conditions in which they are formed. On the other hand, low temperature minerals are much more stable because the surface conditions are much more similar to the conditions in which they occur.
The Bowen's Reaction Continuous and Discontinuous
Discontinuous series starts with olivine, then pyroxene, amphibole and biotite. What makes this a "reaction sequence" rather than an ordinary series is that each mineral in the series is replaced by the next one as the molten cools. As Bowen put it, "The disappearance of minerals in the order they appear is the essence of the reaction series." Olivine forms crystals, which then react with the rest of the parody as pyroxene forms. At a certain point, all olivine is absorbed and found only in pyroxene. The pyroxene then reacts with the liquid and the amphibole crystals replace it and then replace the biotite amphibian.
The continuous series is plagioclase feldspar. At high temperatures, the high-calcium variety anorthite forms. Then as temperatures fall it is replaced by more sodium-rich varieties: bytownite, labradorite, andesine, oligoclase, and albite. As the temperature continues to fall, these two series merge, and more minerals crystallize in this order: Alkali feldspar, muscovite, and quartz.
A minor reaction series involves the spinel group of minerals: chromite, magnetite, ilmenite, and titanite. Bowen placed them between the two main series.

Other Parts of the Series
The entire series is not found in nature, but many magmatic rocks show parts of the sequence. The main limitations are the state of the liquid, the rate of cooling and the tendency of mineral crystals to settle under gravity:
- If the liquid is depleted from an element needed for a particular mineral, the series containing that mineral is discontinued.
- If the magma cools faster than the reaction can continue, the early minerals may remain in partially absorbed form. This changes the evolution of magma.
- If the crystals can rise or sink, they stop reacting with the liquid and settle elsewhere.
Who is Norman L. Bowen ?
Norman Levi Bowen was born June 21, 1887 and death September 11, 1956. He was a Canadian geologist. Bowen "experimental petrology and our understanding of mineral crystallization revolutionized". Initial geology students are familiar with Bowen's reaction series, which shows how different minerals crystallize at different pressures and temperatures.
Source: https://geologyscience.com/geology/bowens-reaction-series/
0 Response to "Bowen s Reaction Series Continuous Vs Discontinuous"
Post a Comment